Dihydrochalcone
Appearance
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Diphenylpropan-1-one | |
| Other names
Hydrochalcone
Benzylacetophenone Hydrocinnamophenone 3-Phenylpropiophenone Phenethyl phenyl ketone Phenyl phenethyl ketone β-Phenylpropiophenone 1,3-Diphenyl-1-propanone ω-Benzyl acetophenone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.150.317 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H14O | |
| Molar mass | 210.27 g/mol |
| Appearance | white solid |
| Density | 1.0614 g/cm3 |
| Melting point | 72–75 °C (162–167 °F; 345–348 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dihydrochalcone (DHC) is the organic compound with the formula C6H5C(O)(CH2)2C6H5. It is the reduced derivative of chalcone (C6H5C(O)(CH)2C6H5). It is a white solid that is soluble in many organic solvents. Dihydrochalcone per se is often minor significance, but some derivatives occur in nature and have attracted attention as drugs.[1]
Natural dihydrochalcones
[edit]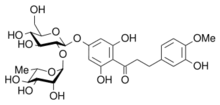
- Aspalathin, a C-linked dihydrochalcone glucoside found in rooibos, a common herbal tea
- Naringin dihydrochalcone, an artificial sweetener derived from naringin
- Neohesperidin dihydrochalcone, an artificial sweetener derived from citrus
- Nothofagin, a C-linked phloretin glucoside found in rooibos
- Phloretin
- Isosalipurpurin
Dihydrochalcones (3′,5′-dihydroxy-2′,4′,6′-trimethoxydihydrochalcone (methyl linderone) and 2′-hydroxy-3′,4′,5′,6′-tetramethoxydihydrochalcone (dihydrokanakugiol) can be found in twigs of Lindera lucida.[2]
References
[edit]- ^ Tomás-Barberán, Francisco A.; Clifford, Michael N. (2000). "Flavanones, Chalcones and Dihydrochalcones - Nature, Occurrence and Dietary Burden". Journal of the Science of Food and Agriculture. 80 (7): 1073–1080. doi:10.1002/(SICI)1097-0010(20000515)80:7<1073::AID-JSFA568>3.0.CO;2-B.
- ^ A dihydrochalcone from Lindera lucida. Yuan-Wah Leong, Leslie J. Harrison, Graham J. Bennett, Azizol A. Kadir and Joseph D. Connolly, Phytochemistry, Volume 47, Issue 5, March 1998, Pp. 891-894, doi:10.1016/S0031-9422(97)00947-3
